PRODUCTS
- INSOL COVID-19 Ag
- RE:FIT® Patch U8
- OraQuick ADVANCE Rapid HIV-1/2 Antibody Test
- INSOL OraQuick HCV Rapid Antibody Test
- Cryosurgical Device Histofreezer
- HIV Oral Fluid Test
- Cryosurgical Device Wartfreezer
- INSOL VTM Viral Transport Medium
- INSOL Saliva DNA Collector
- INSOL Influenza A/B Ag
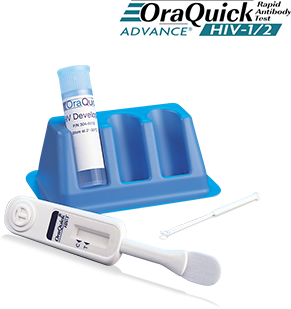
OraQuick ADVANCE Rapid HIV-1/2 Antibody Test
| Product Name | OraQuick ADVANCE Rapid HIV-1/2 Antibody Test |
|---|---|
| Classification | IVD medical device for professional use |
Product Information
- Intended Use
- OraQuick ADVANCE Rapid HIV-1/2 Antibody Test is a single-use immunoassay for the qualitative detection of antibodies to Human lmmunodeficiency Virus Type 1 (HIV-1) and Type 2 (HIV-2) in oral fluid, fingerstick whole blood, venipuncture whole blood and plasma specimens. The product is intended for use as a point-of-care test to aid in the diagnosis of infection with HIV-1 and HIV-2.
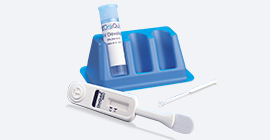
- Kit Components
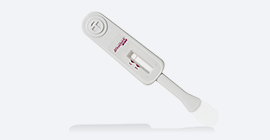
- *Test Device: plastic instrument with flat pad at the bottom and result window in the middle having triangular labels, C (control) and T (test)
- *Developer Solution: liquid buffer in plastic vial
- *Specimen Collection Loop: white plastic rod with round loop attached at the end for specimen collection
- *Test Stand: blue plastic stand with 3 holes for developer solution vials
- Storage Instructions
- *ut in airtight container. Store at 2℃-27℃.
- *Shelf Life: within 30 months from date of manufacturing
- *For immediate use after removed from pouch
Packing Unit : 100kit
Instructions for Use