PRODUCTS
- INSOL COVID-19 Ag
- RE:FIT® Patch U8
- OraQuick ADVANCE Rapid HIV-1/2 Antibody Test
- INSOL OraQuick HCV Rapid Antibody Test
- Cryosurgical Device Histofreezer
- HIV Oral Fluid Test
- Cryosurgical Device Wartfreezer
- INSOL VTM Viral Transport Medium
- INSOL Saliva DNA Collector
- INSOL Influenza A/B Ag

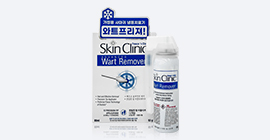
Cryosurgical Device Histofreezer
| Product Name | Cryosurgical Device Histofreezer |
|---|---|
| Type name | Histofreezer Cryosurgical System Model No.H-30 |
| Classification | Medical Device |
| Indications | verruca vulgaris, verruca plana, acrochordon (skin tag), seborrheic keratosis, condyloma accuminata, verruca plantaris, molluscum contagiosum, actinic keratosis, lentigo, and cervical erosion |
Product Information
- Intended Use
- Destroying the wart or other benign lesions through direct application of liquified gas at cryogenic temperature
- Ingredients
- *Dimethyl ether (DME)
- *Propane
- *Isobutane
- Kit Components
- *Aerosol canister: 80ml
- *Applicators: 16 x small 2mm applicators & 16 x medium 5mm applicators
- *Instructions for Use
- Storage Instructions
- *Protect from sunlight. Do not expose to high temperatures exceeding 50˚C.
- *Shelf Life : within 3 years from date of manufacturing
Packing Unit : 1Unit
Instructions for Use